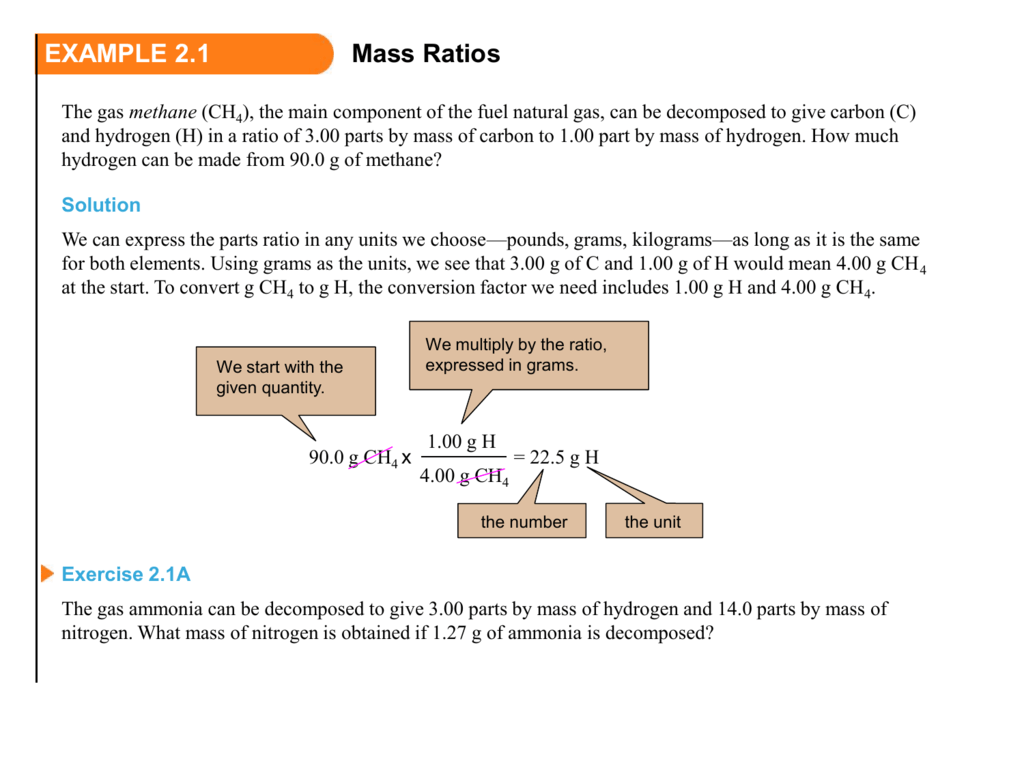
EXAMPLE 2.1 Mass Ratios continued
4: Atomic Structure 4.5: Mass Ratio Calculation

Comment calculer un pourcentage massique 13 étapes
A teaching video on Mass Ratio used in the 'Global Climate Change' module at The University of Texas at Austin.
PPT S’MORES PowerPoint Presentation, free download ID1881651
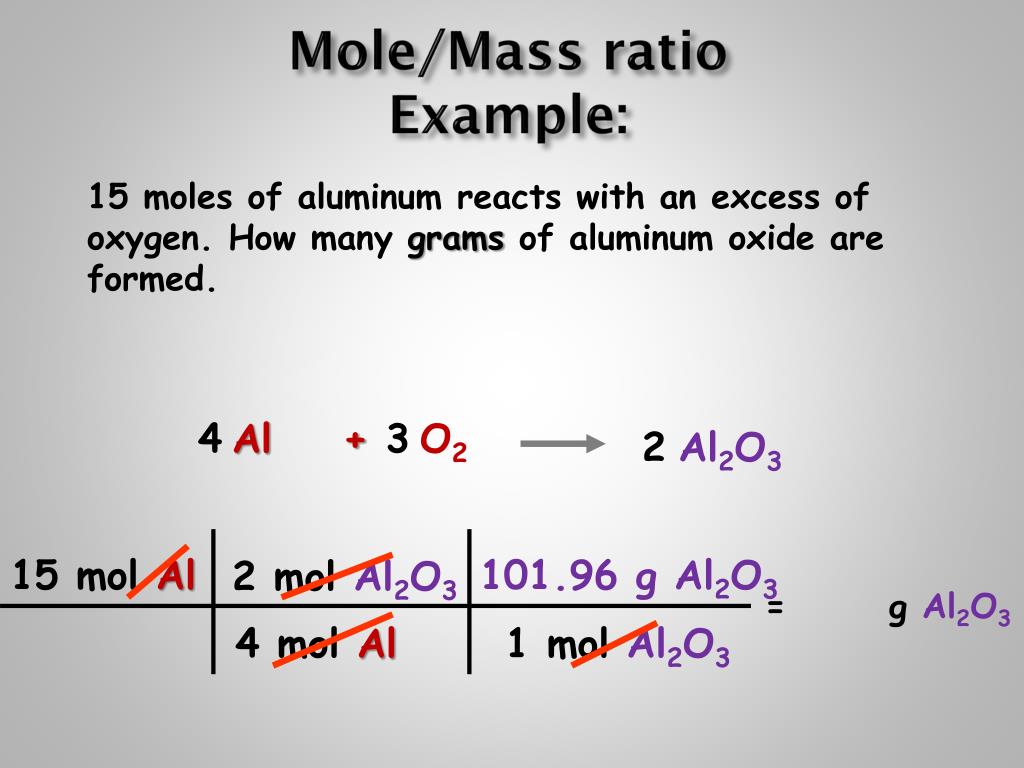
Calculating mole ratios Remember, stoichiometry is the study of mass relations. To master it, you need to be comfortable with unit conversions and balancing equations. From there, the focus is on mole relationships between reactants and products in a chemical reaction. Mass-Mass Stoichiometry Problem
uchopenie ideológie Cena how to calculate mass percent odvolanie vitajte inzerovať
Mass/charge ratio is given the symbol m/z (or sometimes m/e). For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. An ion with a mass of 56 and a charge of 2+ would also have a mass/charge ratio of 28. In the last diagram, ion stream A is most deflected - it will contain ions with the smallest mass.

Mass Ratios YouTube
Mass Ratio Here the ratio of the masses of molecules or atoms can be calculated. Please enter the empirical formula of two molecules, the entry format is similar to the Round to Molecular mass X: u Molecular mass Y: u Examples: The water molecule H 2 O has a mass ratio of oxygen to hydrogen of 7.94.

mass to mass ratio YouTube
We can balance the equation by placing a 2 in front of NaOH (so that there are 2 Na atoms on each side) and another 2 in front of H A 2 O (so that there are 6 O atoms and 4 H atoms on each side). Doing so gives the following balanced equation: 2 NaOH ( a q) + H A 2 SO A 4 ( a q) → 2 H A 2 O ( l) + Na A 2 SO A 4 ( a q)

Charge to mass ratio of electron proof Charge to mass ratio mathematical proof F Sc Chemistry
Mass Ratios, Percent Composition & Empirical Formulas Quiz. This online quiz is intended to give you extra practice in determining mass ratios, percent compositions and empirical formulas of a variety of chemical compounds. Select your preferences below and click 'Start' to give it a try! Number of problems: 1. 5.

Unit 4 Using mass ratios to predict formulas YouTube
In chemistry, the mass fraction of a substance within a mixture is the ratio (alternatively denoted ) of the mass of that substance to the total mass of the mixture. [1] Expressed as a formula, the mass fraction is: Because the individual masses of the ingredients of a mixture sum to , their mass fractions sum to unity:
Mass charge ratio Science, Chemistry, Atoms, Atoms Ions ShowMe
Where: Mass Ratio (MR) is the ratio of the mass of Substance A to the mass of Substance B, typically expressed as a decimal or fraction. Mass of Substance A (MA) is the mass of the first substance in the mixture or reaction, typically measured in grams (g), kilograms (kg), or other appropriate units.

Quantum Gravity and the Holographic Mass Ratio Description The New Energy Industry
The mass-to-charge ratio ( m / Q) is a physical quantity relating the mass (quantity of matter) and the electric charge of a given particle, expressed in units of kilograms per coulomb (kg/C). It is most widely used in the electrodynamics of charged particles, e.g. in electron optics and ion optics .

9th Class Chemistry Chapter 6 Volume over Mass Ratio YouTube
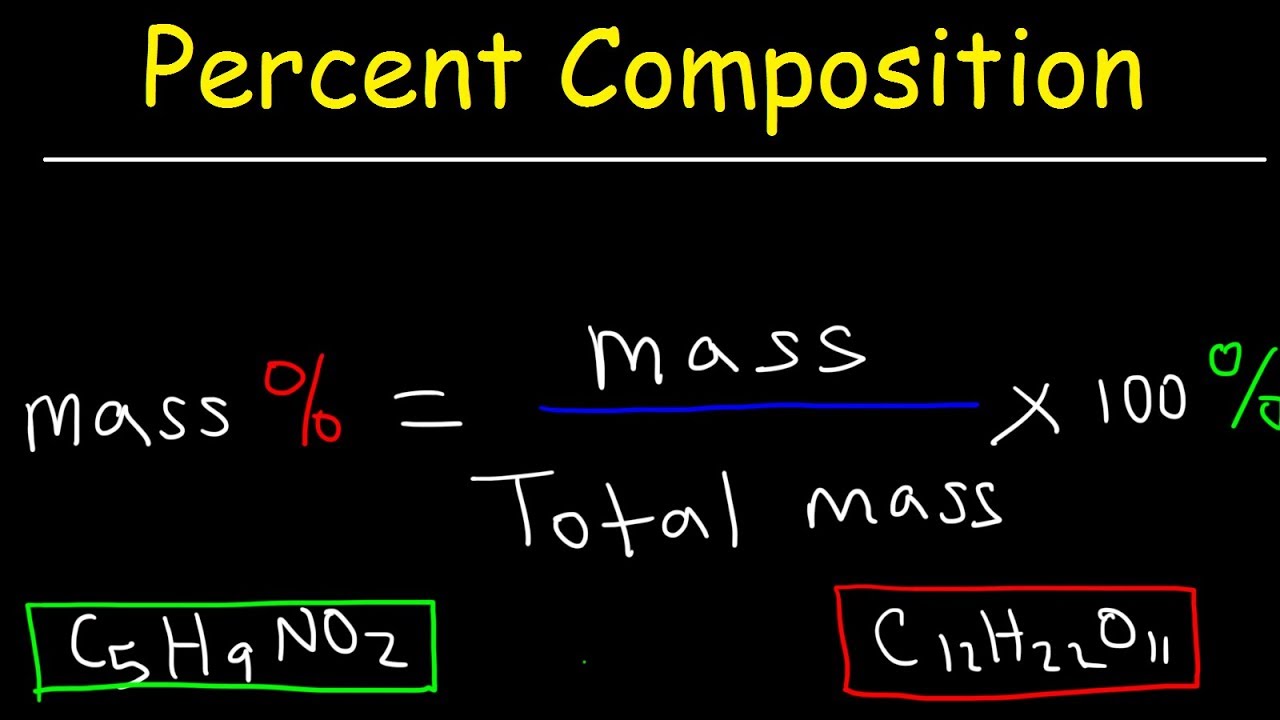
Mass Percent Equations and Indicator Words. The mass percent of a solution is defined as the ratio of the mass of solute that is present in a solution, relative to the mass of the solution, as a whole. Additionally, because this type of concentration, which is typically calculated for solid- and liquid-phase solutions, is expressed as a percentage, this proportion must be multiplied by 100, as.
CHEMISTRY 11 ISOTOPES AND ATOMS October 26, 2010
Mass Ratio Calculation | Chemistry for Non-Majors Uncertainty in Multiplication and Division Uncertainty in Addition and Subtraction Homogenous and Heterogenous Mixtures Study Guides for thousands of courses. Instant access to better grades!

Structure of Atom Class 11 Chemistry Charge to Mass Ratio of Electrons YouTube
In chemistry, mass ratio, often called "percent composition by mass," is the proportion of a particular molecule that consists of each that molecule's constituent elements.

Mass Ratio YouTube
In this Tutoring Thursday session, we discover how to calculate the mass ratio of a chemical compound.We demonstrate simple steps that will allow you to conf.

Charge to Mass Ratio Structure of the Atom CBSE Class 11 Chemistry YouTube
A mass spectrometer ionizes atoms and molecules with a high-energy electron beam and then deflects the ions through a magnetic field based on their mass-to-charge ratios ( m / z ). The mass spectrum of a sample shows the relative abundances of the ions on the y-axis and their m / z ratios on the x-axis. If z = 1

PPT Chemistry Review PowerPoint Presentation, free download ID5678406
10. 50 we havelnm0m=vu0=3×1075×103=6000andm0m=e6000=102600This ratio is so large that much less than one molecule of rocket would be left, regardless of the rocket's initial mass! Chemical rockets do not develop a sufficiently large exhaust velocity to be viable options for interstellar travel.